THE CARNOT CYCLE: A FUNDAMENTAL CONCEPT IN THERMODYNAMICS-II
The Carnot Cycle: A Fundamental Concept in Thermodynamics-II
In our previous article, we introduced you to the Carnot cycle, a fundamental concept in thermodynamics. Now, we delve deeper into this essential concept to understand its key points and applications.
Recap: What is the Carnot Cycle? The Carnot cycle is an idealized thermodynamic cycle that sets the upper limit on the efficiency of heat engines. It was developed by French physicist Sadi Carnot in 1824 and forms the foundation for understanding the principles of heat engines and refrigerators.
Key Points in the Carnot Cycle:
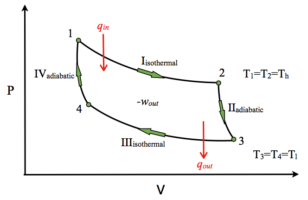
- Four Reversible Processes: The Carnot cycle consists of four reversible processes: isothermal expansion, adiabatic expansion, isothermal compression, and adiabatic compression. These processes involve the working substance, typically a gas, going through a series of state changes.

- Two Isothermal and Two Adiabatic Processes: During the isothermal expansion and compression processes, the working substance exchanges heat with a hot and a cold reservoir, respectively. These processes occur at constant temperature, resulting in maximum efficiency. In contrast, the adiabatic expansion and compression processes involve no heat transfer and are responsible for the change in internal energy.
- Efficiency Maximization: The primary purpose of the Carnot cycle is to maximize the efficiency of heat engines. It serves as an idealized benchmark for real-world engines, illustrating that no engine can be more efficient than a Carnot engine operating between the same temperature reservoirs. The efficiency of a Carnot engine depends solely on the temperature difference between the hot and cold reservoirs.
- Second Law of Thermodynamics: The Carnot cycle is closely related to the second law of thermodynamics, which states that no engine can operate with 100% efficiency. The cycle demonstrates that some energy will always be lost as waste heat in real-world engines.
- Refrigeration and Heat Pumps: While the Carnot cycle is mainly associated with heat engines, it is equally applicable to refrigeration and heat pump systems. In these cases, the cycle is reversed, and work is done to transfer heat from a colder reservoir to a hotter one. The coefficient of performance (COP) is used to measure the effectiveness of refrigeration and heat pumps.

- Entropy and Reversibility: The Carnot cycle is also essential for understanding the concept of entropy. It highlights the idea that the cycle is reversible, meaning it can operate in both directions, and entropy remains constant in a reversible process. In real-world, irreversible processes, entropy increases, leading to a decrease in efficiency.
- Practical Applications: While real engines and refrigeration systems do not achieve the theoretical efficiency of the Carnot cycle, it provides a crucial reference point for engineers and scientists when designing and improving these systems. Efforts are made to make engines and refrigeration systems more efficient and to reduce energy waste.
In conclusion, the Carnot cycle is a fundamental concept in thermodynamics that plays a vital role in understanding the limits of efficiency in heat engines, refrigeration systems, and heat pumps. It serves as a benchmark for evaluating the performance of these systems and provides a foundation for the second law of thermodynamics. While real-world systems cannot achieve the perfect efficiency of the Carnot cycle, it remains an essential theoretical concept in the field of thermodynamics.